Investigator-
Initiated Study
Evaluation Process
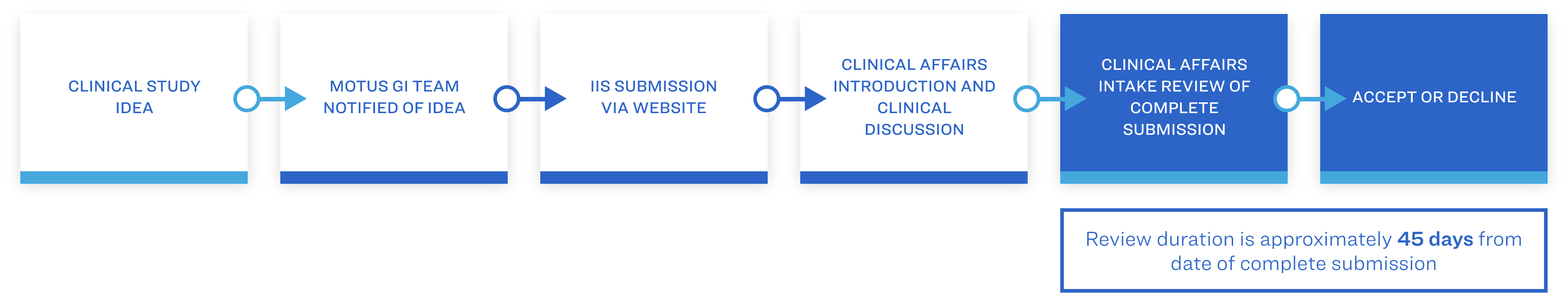
Each full submission will consist of the completion of the IIS Study Application Form, study design and study budget, which will initially be reviewed by the Clinical Affairs Department. Once approved, the submission will progress to the Investigator-Initiated Study (IIS) Board Review where it will receive a final decision. This decision will be communicated to the IIS investigator.
In addition to meeting ethical and scientific standards, submissions will be reviewed for alignment to the Motus GI’s Strategic Focus.
Additional Resources
IIS Application Form
ClinicalTrials.gov
Good Clinical Practice (GCP)
AdvaMed Code of Ethics

Tracking and Monitoring Systems
Motus GI will monitor and audit IISs and their adherence to Motus GI policies and standards. In addition, IIS investigators’ compliance and adherence to their contractual obligations related to disclosure of IIS findings, agreed upon milestones, and safety information reporting will also be monitored.
