Mission
Motus GI® provides innovative solutions to address unmet needs in GI endoscopy, cost-effectively improving clinical outcomes that benefit patients, providers and payers worldwide.
Core Values
We Value Our Team and Their Diversity.
We Are Committed to Improving Patients’ Lives.
We Instill Care and Quality in All We Do.
We Persevere to Deliver All of Our Commitments.
Company Overview
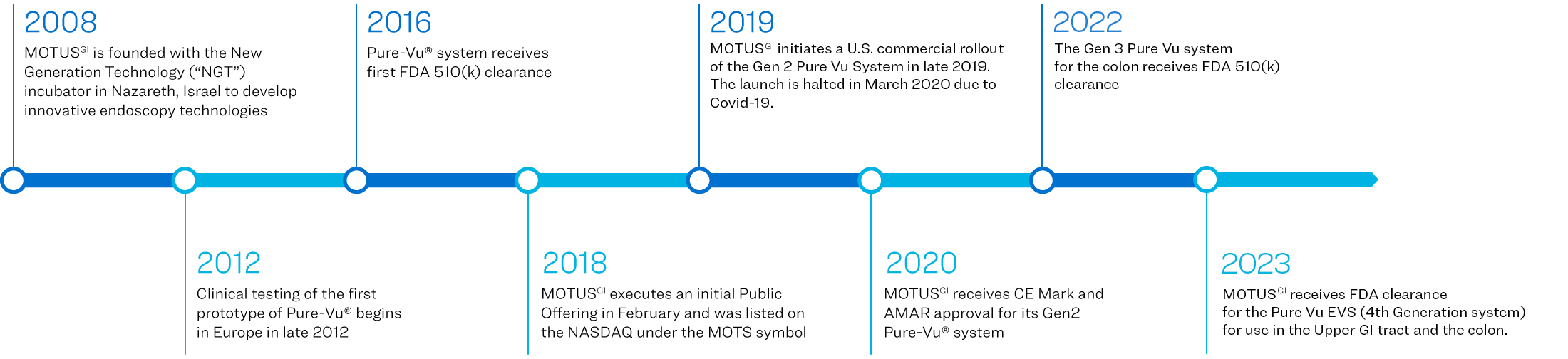
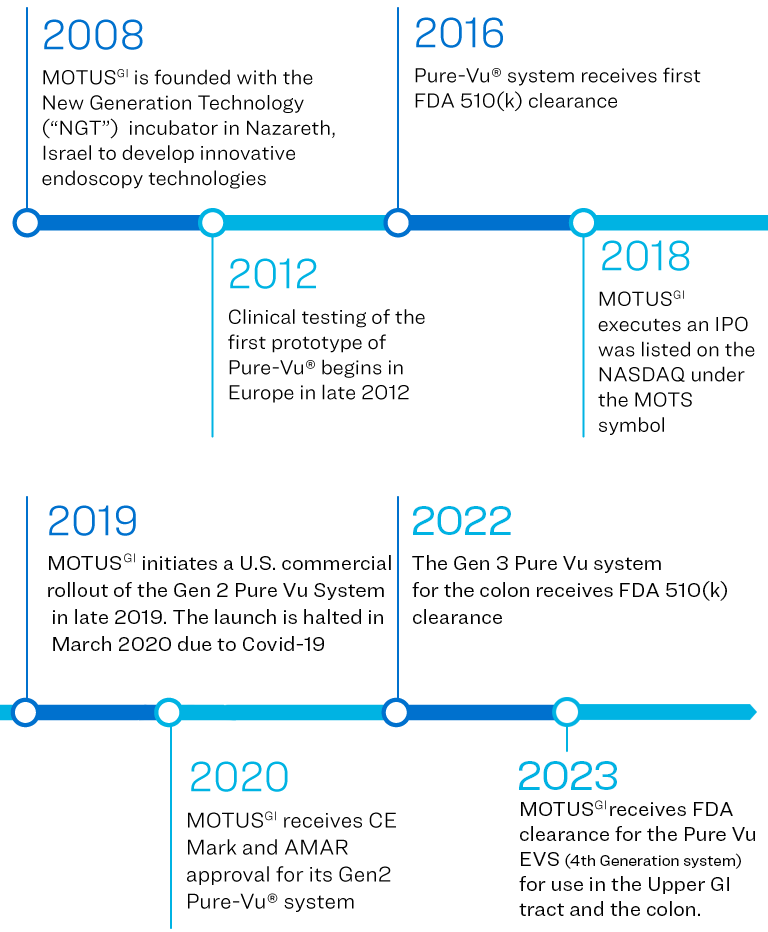
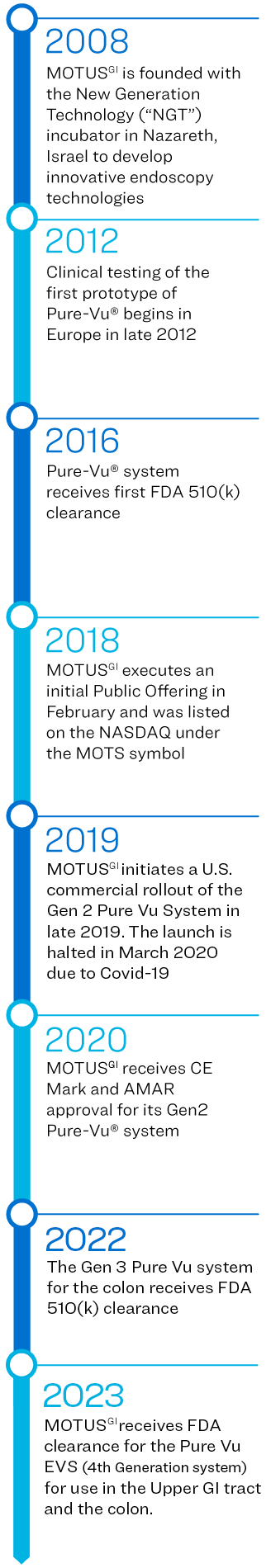
Motus GI Holdings, Inc. (the “Company”) was incorporated in Delaware, U.S.A. in September 2016. The Company and its subsidiaries, Motus GI Technologies, Ltd. and Motus GI, LLC., are collectively referred to as “Motus GI” or the “Company”.
The Company has developed the Pure-Vu® System, a medical device that has been cleared by the U.S. Food and Drug Administration (the “FDA”) to help facilitate the cleansing of the gastrointestinal tract to improve visualization during in an upper GI endoscopy (EGD) or a colonoscopy. The Pure-Vu® System has received a CE Mark in the EU for use in colonoscopy. The Pure-Vu® System integrates with standard and slim colonoscopes, as well as gastroscopes, to improve visualization during colonoscopy and upper GI procedures while preserving established procedural workflow and techniques. Through irrigation and evacuation of debris, the Pure-Vu® System is designed to provide better-quality exams. The Company began market introduction in the U.S. of it's fourth Generation system the Pure-Vu® EVS, which received FDA clearance in Q4 2023 for use in both the upper and lower GI tract and is currently targeting early adopter hospitals.